CE Mark Testing & Certification
The CE mark is a mandatory European marking for certain product groups to indicate conformity with the essential requirements set out in European Directives. In order to use the CE mark on a product, the manufacturer must draw up an EU declaration of conformity (DoC) in which the manufacturer attests to conformity with all relevant New Approach Directives (NADs) and takes sole legal responsibility. In some instances, a NAD may require a Notified Body to issue a Certificate of EU declaration of conformity (DoC) Conformity in order to verify the performance of the product or constancy of the production process (Factory Production Control, for example).
F2 Labs can assist you in identifying the requirements for CE certification. We have a staff of experts experienced in the CE mark who are ready to assist you throughout the process of CE testing and CE certification. With more than 20 years of experience in CE testing and evaluation, F2 Labs has helped many businesses successfully enter into the European and global markets with CE certification.
We, at our CE Mark Testing Lab test products to the following directives:
- Radio Equipment Directive 2014/53/EU (RED), formerly R&TTE Directive
- EMC Directive 2014/30/EU (formerly Directive 2004/108/EC)
- (ATEX) Directive 2014/34/EU (formerly Directive 94/9/EC)
- General Product Safety Directive 2001/95/EC (this Directive does not involve applying a CE mark but can be applicable to your product)
- Low Voltage Directive 2014/35/EU (formerly Directive 2006/95/EC)
- Machinery Directive 2006/42/EC
- Medical Devices 93/42/EEC
Steps to Obtaining the CE Mark
F2 Labs will walk you through each step of the CE testing certification process:
- Identify the directive(s) and harmonized standards applicable to the product
- Verify the product-specific requirements
- Identify whether a Notified body approval is required
- Test the product to check its conformity
- Compile and keep available the required technical documentation
- Affixation of the CE mark to your product and complete an EU declaration of conformity
The EU declaration of conformity (DoC) must include: manufacturer’s details (name, address, etc.); description of the product; List of EU Directives to which the product complies; list of relevant EN standards used; must be signed and dated by an authorized representative of the company placing the product on the European Market, and must be translated into the native language of the country in which it will operate.
The CE mark is covered by Council Decision 93/465/EC. Annex B(d) provides the following guidelines:
- The CE conformity marking must consist of the initials ‘CE’ taking the following form:
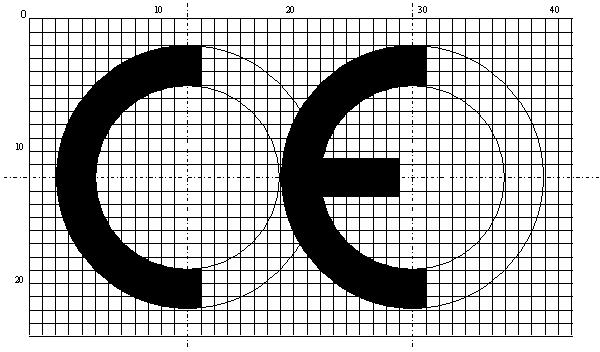
- If the CE conformity marking is reduced or enlarged the proportions must be kept the same.
- Where the directive concerned does not state specific dimensions, the CE marking must have a height of at least 5 mm.
- The CE marking must be affixed to the product or to its data plate. However, if that is not possible or warranted on account of the nature of the product, it must be affixed to the packaging, if any, and to the accompanying documents, where the directive concerned provides for such documents.
- The CE marking must be affixed visibly, legibly, and indelibly.
Helpful Links:
CE Mark Testing Lab
EU Directive Testing – F2 Labs has more than 20 years of experience in assisting our clients with CE testing certification, European product compliance evaluation and testing to the applicable EN standards under the EU Directives so that they can obtain the CE mark for their product(s).
EMC Directive 2014/30/EU (formerly Directive 2004/108/EC) -Our CE Mark Testing Lab can perform Electromagnetic Compatibility (EMC/EMI) testing of your product at our facility or on-site at your facility.
Low Voltage Directive 2014/35/EU (formerly Directive 2006/95/EC) – F2 Labs can provide the testing services you need in order to prove that your product is compliant with the Low Voltage Directive.
Machinery Directive 2006/42/EC Evaluation & Testing – F2 Labs can perform the evaluation and CE certification testing required by the Machinery Directive at our facility or yours.
EU Authorized Representative – F2 Labs offers can serve as your Authorized EU Representative serving as the contact point.
Medical Device Directive 93/42/EEC – Manufacturers must comply with the medical device directive in order to sell their medical products into Europe.
Radio Equipment Directive 2014/53/EU (RED), formerly R&TTE Directive – F2 Labs can assist you with your Radio Equipment Directive 2014/53/EU (RED), formerly R&TTE Directive, testing needs.
General Product Safety Directive 2001/95/EC – Although this Directive does not involve CE marking the product, it may be a requirement for your European compliance project. Our CE Mark Testing Lab can assist you with testing required for compliance with the General Product Safety Directive.
WEEE Directive 2012/19/EU Testing – F2 Labs sales and engineering staff is always available to assist you with your European projects. We can talk with you regarding these WEEE disposal laws and offer our best guidance.
RoHS Directive 2011/65/EU – RoHS is a CE marking Directive and if your product is within its scope then it must carry a CE marking and be referenced on an accompanying declaration of conformity.
Food Contact Materials Regulation (EC) 1935/2004 – The European Union has specific legislation for equipment and products that come into contact with food and it has its own mark. F2 Labs can assist you with the compliance requirements for this regulation.
Frequently Asked Questions
What is the CE Mark?
The CE mark is a mandatory European marking indicating that a product conforms to the essential requirements set out in European Directives. It signifies compliance with EU safety, health, and environmental protection standards.
What Products Require the CE Mark?
The CE mark is required for certain product groups within the European Union. This includes products that fall under directives such as Radio Equipment, EMC, ATEX, Low Voltage, Machinery, and Medical Device Directive.
What is an EU Declaration of Conformity (DoC)?
An EU Declaration of Conformity (DoC) is a document in which the manufacturer attests to conformity with all relevant New Approach Directives (NADs). It is a mandatory step for affixing the CE mark, where the manufacturer takes sole legal responsibility for compliance.
When is a Notified Body Required for CE Certification?
A Notified Body may be required in instances where a New Approach Directive (NAD) necessitates third-party verification of the product’s performance or production process consistency. This could include Factory Production Control or other specific assessments.
What are the Steps to Obtaining the CE Mark?
The steps include identifying applicable directives and standards, verifying product-specific requirements, determining if Notified Body approval is needed, product testing for conformity, compiling technical documentation, affixing the CE mark, and completing an EU DoC.
What Should be Included in the EU Declaration of Conformity?
The EU DoC must include the manufacturer’s details, a product description, a list of EU Directives and EN standards complied with, and must be signed and dated by an authorized representative. It should also be translated into the native language of the country where the product will operate. See the specific applicable Directives for the exact requirements.
How Should the CE Mark Be Affixed?
The CE mark must be affixed visibly, legibly, and indelibly to the product or its data plate. If not possible, it should be on the packaging and accompanying documents. The mark should maintain specific proportions and have a minimum height of 5mm if no specific dimensions are stated in the directive.
How Can F2 Labs Assist with CE Certification?
F2 Labs offers expertise in CE testing and evaluation services, helping businesses to navigate the process for successful entry into European and global markets. With over 205 years of experience, our team can assist in identifying requirements, testing products, and ensuring all steps for CE certification are completed efficiently.

