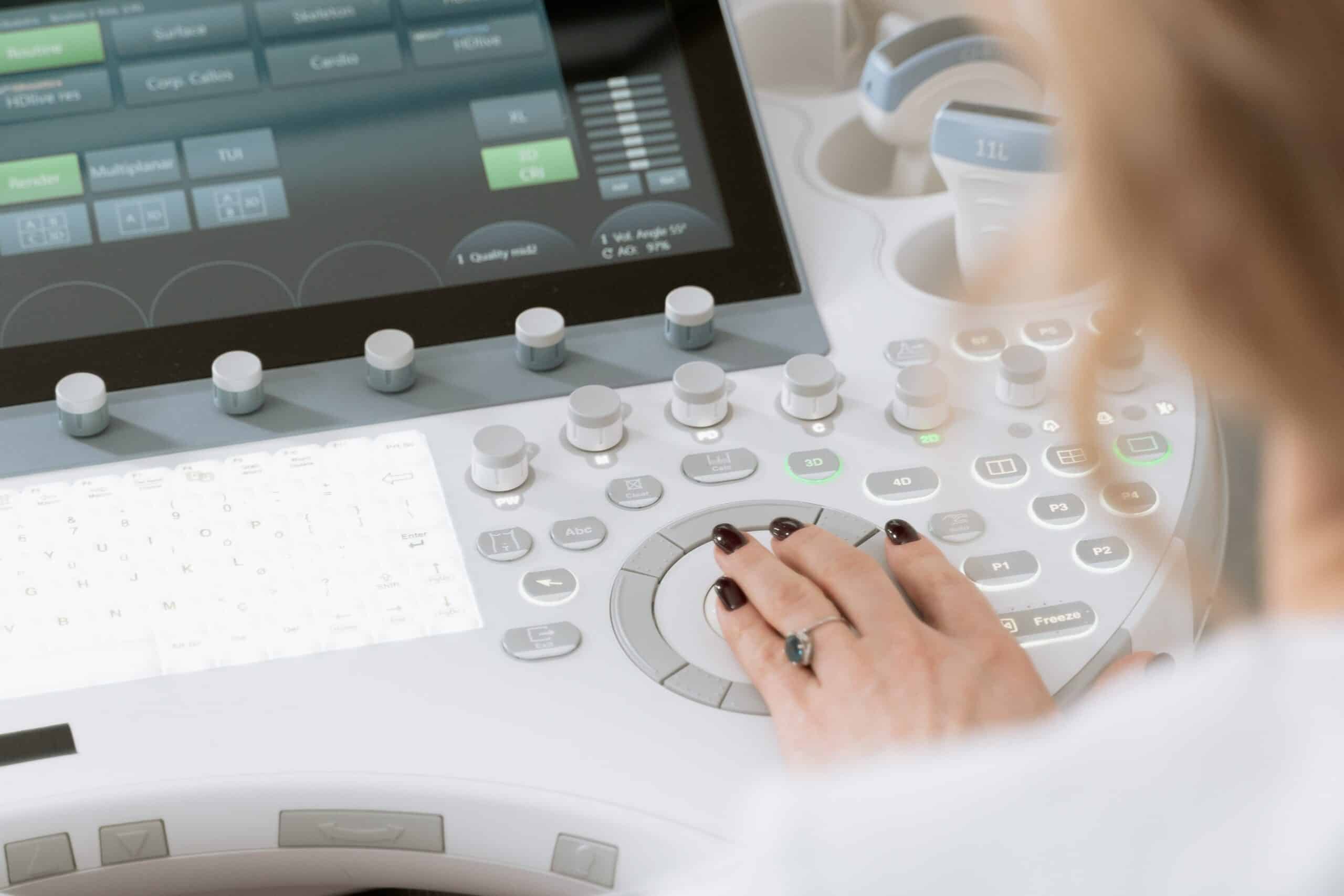
F2 Labs provides specialized medical device EMC testing services to ensure compliance with FDA, IEC 60601, and international regulatory standards. Our experts help you achieve safe, reliable device performance in even the most demanding electromagnetic environments.
What is Medical Device EMC Testing ?
Medical device EMC testing ensures that medical electrical equipment performs reliably in real-world electromagnetic environments—without emitting harmful interference or being susceptible to external electromagnetic disturbances. This testing covers both emissions and immunity (including conducted, radiated, and electrostatic discharge) to meet regulatory requirements and safeguard patient and operator safety.
Why EMC Testing Matters for Medical Devices
The Medical Device EMC Testing Process
Pre‑compliance Scans
Pre-compliance scans are essential for early detection of design issues before full testing. These scans help identify potential electromagnetic compatibility problems, allowing for corrective actions during the development phase. This proactive approach not only saves time and costs but also enhances the reliability of the device by addressing issues early.
Emissions Testing
Emissions testing measures unintentional electromagnetic emissions, both radiated and conducted. This key step in EMC testing ensures that the device does not emit electromagnetic interference that could affect other equipment. By adhering to international emissions standards, devices can safely coexist with other electronic products in healthcare environments.
Immunity Testing
Immunity testing verifies a device’s resilience to various electromagnetic disturbances, such as electrostatic discharge (ESD), radiated fields, and voltage dips. This testing ensures that devices maintain functionality and safety standards even in the presence of electromagnetic interference, crucial for patient safety and device reliability.
Reporting
Manufacturers must clearly document essential performance, immunity pass/fail criteria, test configurations, allowances/deviations, and any modifications made to pass EMC testing. Labeling should include EMC compliance levels, intended use environments, behavior under interference, and accessory compatibility
These enhancements ensure that your medical device meets all necessary standards for electromagnetic compatibility, paving the way for successful market entry and patient safety. Let F2 Labs guide you through the complex landscape of EMC testing and certification.
Ensuring Compliance and International Access
Read more about our medical device testing:
F2 Labs is an industry leader in medical device safety testing helping you bring your medical device to market utilizing the IEC 60601 series of standards performed in our medical device testing lab. With our team of experts, we can help you navigate the compliance maze to gain entry into North America, Europe, and beyond. We provide design assistance as well as comprehensive testing to the 60601/80601 series of standards including any applicable Collateral (60601-1-X) or Particular (60601-2-X) standards. Let F2 Labs be your compliance partner throughout this process.
Medical Device Safety Testing Standards
ASCA Program (FDA)
The Accreditation Scheme for Conformity Assessment (ASCA) program is designed to increase both the manufacturer and consumer confidence in medical device safety testing. Although it does not introduce new requirements for products to be compliant, it does certify the laboratories testing with an initial application process and subsequent periodic audits. Learn More
Essential Performance
In order for your medical device to comply with IEC 60601-1, a risk assessment is performed. In accordance with that risk assessment, you must determine your medical device’s essential performance or at what point device failure would result in an unacceptable risk. Learn More
Medical Device Regulation
Our Medical device testing lab utilizes this revised regulation for medical devices which replaces three current medical device directives: the In Vitro Diagnostic Medical Devices Directive 98/79/EC (IVDMD), the Medical Devices Directive 93/42/EEC (MDD), and the Active Implantable Medical Devices Directive 90/385/EEC (AIMDD). Learn More
IEC 60601-1
3rd edition – This standard is for medical electrical equipment and focuses on general requirements for basic safety and essential performance. Certain aspects of safety and performance are defined by the collateral standards, such as IEC 60601-1-11 for home healthcare equipment; IEC 60601-1-6 for usability; IEC 60601-1-8 for alarms; Risk Assessment to ISO 14971; and more. Learn More
Medical Device EMC Testing Standards
AIM 7351731 Testing
AIM 7351731 specifically applies to medical electrical equipment and systems and focuses on the most common RFID technologies, which are identified in our medical device testing lab, to be currently deployed in healthcare and other environments where RFID Readers may be used but never seen. Learn MoreASCA Program (FDA)
The Accreditation Scheme for Conformity Assessment (ASCA) program is designed to increase confidence in medical device EMC testing (by manufacturers and consumers). Although it does not introduce new requirements for products to be compliant, it does certify the laboratories testing with an initial application process and subsequent periodic audits. Learn MoreIEC 60601-1-2
4th edition – this standard is for medical electrical equipment and focuses on electromagnetic disturbances. In the EU, medical devices are required to comply with the 4th edition of EN 60601-1-2. There is no allowance for legacy devices, as is allowed by the FDA. Learn MoreOther Immunity Testing (FDA)
In addition to AIM 7351731, the FDA almost always also requires testing to ensure other types of devices won’t interfere with a product’s function. This may just require information with your pre-market submission, but the FDA may request additional information. Learn MoreWireless Coexistence Testing
Wireless Coexistence testing is what the FDA stipulates for medical devices that contain wireless transmitters. This standard is used in medical device testing lab to determine if a product is affected by unintended signals being used in the same operating environment. Learn MoreCommon Collateral & Particular Standards for Medical Device Testing
F2 Labs can perform evaluations to IEC 60601-1, titled “Medical Electrical Equipment – Part 1: General requirements for basic safety and essential performance”
IEC 60601-1 Scope
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of
MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS hereafter referred to as
ME EQUIPMENT and ME SYSTEMS.
If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only or to
ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the
case, the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS, as relevant.
HAZARDS inherent in the intended physiological function of ME EQUIPMENT or ME SYSTEMS within
the scope of this standard is not covered by specific requirements in this standard except in 7.2.13 and 8.4.1.
NOTE 1 See also 4.2.
The IEC 60601 series does not apply to:
- in vitro diagnostic equipment that does not fall within the definition of ME EQUIPMENT, which is covered by the IEC 61010 series [61];
- implantable parts of active implantable medical devices covered by the ISO 14708 series [69]; or
- medical gas pipeline systems are covered by ISO 7396-1 [68].
NOTE 2 ISO 7396-1 applies the requirement of IEC 60601-1-8 to certain monitoring and ALARM SIGNALS.
IEC 60601-1-2 – Medical electrical equipment – Part 1-2: General requirements for basic safety and essential performance – Collateral Standard: Electromagnetic disturbances – Requirements and tests
1.1 * Scope This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS hereafter referred to as ME EQUIPMENT and ME SYSTEMS. This collateral standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of ME EQUIPMENT and ME SYSTEMS in the presence of ELECTROMAGNETIC DISTURBANCES and to ELECTROMAGNETIC DISTURBANCES emitted by ME EQUIPMENT and ME SYSTEMS. BASIC SAFETY with regard to ELECTROMAGNETIC DISTURBANCES is applicable to all ME EQUIPMENT and ME SYSTEMS.
F2 Labs can perform evaluations to IEC 60601-1-6 Edition 3.0 titled “Medical electrical equipment – Part 1-6: General requirements for basic safety and essential performance – Collateral standard: Usability”
IEC 60601-1-6 Scope
This International Standard specifies a PROCESS for a MANUFACTURER to analyze, specify, design, VERIFY and VALIDATE USABILITY, as it relates to BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT, hereafter referred to as ME EQUIPMENT.
This USABILITY ENGINEERING PROCESS assesses and mitigates RISKS caused by USABILITY problems associated with CORRECT USE and USE ERRORS, i.e., NORMAL USE. It can be used to identify but does not assess or mitigate RISKS associated with ABNORMAL USE.
If the USABILITY ENGINEERING PROCESS detailed in this collateral standard has been complied with and the acceptance criteria documented in the USABILITY VALIDATION plan have been met (see 5.9 of IEC 62366:2007), then the RESIDUAL RISKS, as defined in ISO 14971, associated with USABILITY of ME EQUIPMENT is presumed to be accepted unless there is OBJECTIVE EVIDENCE to the contrary (see 4.1.2 of IEC 62366:2007).
F2 Labs can perform evaluations to IEC 60601-1-8 Edition 2.1 titled “Medical electrical equipment – Part 1-8: General requirements for basic safety and essential performance – Collateral standard: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems.”
IEC 60601-1-8 Scope
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS hereafter referred to as ME EQUIPMENT and ME SYSTEMS.
This collateral standard specifies requirements for ALARM SYSTEMS and ALARM SIGNALS in ME EQUIPMENT and ME SYSTEMS.
It also provides guidance for the application of ALARM SYSTEMS.
F2 Labs can perform evaluations to IEC 60601-1-9 1st Edition titled “General requirements for basic safety and essential performance – Collateral Standard: Requirements for environmentally conscious design”
IEC 60601-1-9 Scope
This International Standard applies to the reduction of adverse ENVIRONMENTAL IMPACTS of MEDICAL ELECTRICAL EQUIPMENT, hereafter referred to as ME EQUIPMENT.
MEDICAL ELECTRICAL SYSTEMS are excluded from the scope of this collateral standard.
F2 Labs can perform evaluations to IEC 60601-1-11 Edition 2.0 titled “Medical electrical equipment – Part 1-11: General requirements for basic safety and essential performance – Collateral Standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment”
IEC 60601-1-11 Scope
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMS for use in the HOME HEALTHCARE ENVIRONMENT, as defined in 3.1, and specified by the MANUFACTURER in the instructions for use. This International Standard applies regardless of whether the ME EQUIPMENT or ME SYSTEM is intended for use by a LAY OPERATOR or by trained healthcare
personnel.
The HOME HEALTHCARE ENVIRONMENT includes:
- the dwelling place in which a PATIENT lives;
- other places where PATIENTS are present both indoors and outdoors, excluding professional healthcare facility environments where OPERATORS with medical training are continually available when PATIENTS are present.
This International Standard does not apply to ME EQUIPMENT and ME SYSTEMS intended solely for use in the EMERGENCY MEDICAL SERVICES ENVIRONMENT, covered by IEC 60601-1-12 or solely for use in professional healthcare facilities covered by IEC 60601-1 without the additions of IEC 60601-1-12 or this collateral standard. Nonetheless, ME EQUIPMENT or ME SYSTEMS can be intended for multiple user environments, and as such, if also intended for use in the HOME HEALTHCARE ENVIRONMENT, are within the scope of this standard.
EXAMPLE ME EQUIPMENT or ME SYSTEMS intended for both the HOME HEALTHCARE ENVIRONMENT and the professional healthcare facility environment.
NOTE HOME HEALTHCARE ENVIRONMENT ME EQUIPMENT and ME SYSTEMS can frequently be used in locations with
unreliable electrical sources and poor electrical grounding.
F2 Labs can perform evaluations to IEC 60601-2-3 Edition 3.0 titled “Medical electrical equipment – Part 2-3: Particular requirements for the basic safety and essential performance of short-wave therapy equipment”
IEC 60601-2-3 Scope
Replacement:
This particular standard specifies the requirements for the safety of SHORT-WAVE THERAPY EQUIPMENT, hereafter referred to as ME EQUIPMENT, as defined in subclause 201.3.206.
LOW POWER EQUIPMENT as defined in subclause 201.3.202 is exempted from certain requirements of this standard.
F2 Labs can perform evaluations to IEC 60601-2-10 Edition 2.0 titled “Medical electrical equipment – Part 2-10: Particular requirements for the basic safety and essential performance of nerve and muscle stimulators”
IEC 60601-2-10 Scope
Replacement:
This International Standard specifies the requirements for the safety of nerve and muscle STIMULATORS, defined in subclause 201.3.204, for use in the practice of physical medicine, hereinafter referred to as ME EQUIPMENT. This includes transcutaneous electrical nerve STIMULATORS (TENS) and electrical muscle STIMULATORS (EMS).
NOTE: A muscle STIMULATOR may also be known as a neuromuscular STIMULATOR.
The following ME EQUIPMENT is excluded:
- ME EQUIPMENT intended to be implanted or to be connected to implanted electrodes;
- ME EQUIPMENT intended for the stimulation of the brain (e.g. electroconvulsive therapy ME EQUIPMENT);
- ME EQUIPMENT intended for neurological research;
- external cardiac pacemakers (see IEC 60601-2-31);
- ME EQUIPMENT intended for averaged evoked potential diagnosis (see IEC 60601-2-40);
- ME EQUIPMENT intended for electromyography (see IEC 60601-2-40);
- ME EQUIPMENT is intended for cardiac defibrillation (see IEC 60601-2-4).
201.12 Accuracy of controls and instruments and protection against hazardous outputs
Clause 12 of the general standard applies except as follows.
201.12.1 Accuracy of controls and instruments
Additional subclauses:
201.12.1.101 * Output amplitude
A means shall be provided to control the STIMULATOR output from minimum to maximum continuously, or in discrete increments of not more than 1 mA or 1 V per increment. At its minimum setting, the output shall not exceed 2 % of that available at the maximum setting of the control.
Compliance is checked by inspection and measurement using the load impedance which is the least favorable within the load impedance range specified in the ACCOMPANYING DOCUMENTS.
201.12.1.102 * PULSE parameters
The values of PULSE DURATIONS, PULSE repetition frequencies, and amplitudes, including any d.c. component, whether caused by an offset or by an unsymmetrical waveform, as described in the ACCOMPANYING DOCUMENTS or indicated on the ME EQUIPMENT (see 201.7.9.2), shall not deviate by more than ± 20 % when measured with a load resistance within the range specified in the ACCOMPANYING DOCUMENTS (see 201.7.9.3).
Compliance is checked by measurement with an error not exceeding ± 10 %.
201.17 Electromagnetic compatibility of ME EQUIPMENT and ME SYSTEMS
Clause17 of the general standard applies.
202 Electromagnetic compatibility – Requirements and tests
IEC60601-1-2:2007 applies except as follows:
202.6.1 Emissions
202.6.1.1.2 Tests
a) Patient cables
Addition:
Connect all relevant electrodes to the contents of a 1-liter capacity phantom filled with 0,9 % saline. Position the phantom within 0,4 m of the ME EQUIPMENT as shown in Figure 202.101.
202.6.2 * Immunity
202.6.2.1.5 PATIENT-COUPLED ME EQUIPMENT and ME SYSTEMS
Addition:
Connect all relevant electrodes and apply them to the contents of a 1-liter capacity phantom filled with 0,9 % saline. Position the phantom within 0,4 m of the ME EQUIPMENT as shown in Figure 202.101.
F2 Labs can perform evaluations to IEC 60601-2-11 Edition 3.0 titled “Medical electrical equipment – Part 2-11: Particular requirements for the basic safety and essential performance of gamma beam therapy equipment”
IEC 60601-2-11 Scope
Replacement:
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of GAMMA BEAM THERAPY EQUIPMENT, including MULTI-SOURCE STEREOTACTIC RADIOTHERAPY equipment, hereafter referred to as ME EQUIPMENT.
If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case, the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS, as relevant.
HAZARDS inherent in the intended physiological function of ME EQUIPMENT or ME SYSTEMS within the scope of this standard are not covered by specific requirements in this standard except in 7.2.13 and 8.4.1 of the general standard.
NOTE See also 4.2 of the general standard.
201.4 General requirements
Clause 4 of the general standard applies.
9 Accuracy of controls and instruments and protection against hazardous outputs
In addition to the requirements of 12.2 of the general standard, when performing the USABILITY ENGINEERING PROCESS, the RISKS associated with USABILITY in the HOME HEALTHCARE ENVIRONMENT for OPERATOR PROFILES including a LAY OPERATOR shall include consideration of at least:
- changes of controls;
- unexpected movement;
- potential for misconnection;
- potential for improper operation, or unsafe use;
- potential for confusion as to current operational mode;
- change in the transfer of energy or substance;
- exposure to environmental conditions specified in this standard;
- exposure to biological materials; and
- small parts being inhaled or swallowed.
Particular emphasis shall be placed on the limited training of a LAY OPERATOR with respect to the ability to intervene and maintain BASIC SAFETY and ESSENTIAL PERFORMANCE. The MANUFACTURER shall include the least capable intended LAY OPERATOR or LAY RESPONSIBLE ORGANIZATION in the USABILITY ENGINEERING PROCESS. EXAMPLES LAY OPERATORS with sensory, cognitive, physical limitations, or comorbidities.
Compliance is checked by inspection of the USABILITY ENGINEERING FILE.
201.12 Accuracy of controls and instruments and protection against hazardous outputs
Clause 12 of the general standard does not apply.
NOTE This is covered by 201.10, IEC 60601-1-6, and IEC 60601-1-8.
201.17 Electromagnetic compatibility of ME EQUIPMENT and ME SYSTEMS
Clause 17 of the general standard applies.
F2 Labs can perform evaluations to IEC 60601-2-18 Edition 3.0 titled “Medical electrical equipment – Part 2-18: Particular requirements for the basic safety and essential performance of endoscopic equipment”
IEC 60601-2-18 Scope
Replacement:
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of ENDOSCOPIC EQUIPMENT together with its INTERCONNECTION CONDITIONS and INTERFACE CONDITIONS.
201.4.3 ESSENTIAL PERFORMANCE
Addition:
201.4.3.101 * Additional ESSENTIAL PERFORMANCE requirements
Additional ESSENTIAL PERFORMANCE requirements are found in the subclauses listed in
Table 201.101.
| Subclause | Requirement |
| To that, there is no unacceptable RISK if the view observed by the OPERATOR has an unexpected image orientation. | Applicability and condition as defined by the manufacturer |
| To ensure that there is no unacceptable RISK if there is a lack of, or a significant error in, provision of a particular spectral output or frequency necessary to provide accurate diagnosis or therapy, which is not identifiable by a trained OPERATOR. | 201.12.1.1 < |
| To ensure that there is no unacceptable RISK that the OPERATOR is viewing the live image during an endoscopic procedure, rather than a recorded image. | 201.13.1.101 |
NOTE See 201.7.9.2.2 g) for warning and safety notices related to the ESSENTIAL PERFORMANCE requirements.
F2 Labs can perform evaluations to IEC 60601-2-23 Edition 3.0 titled “Medical electrical equipment – Part 2-23: Particular requirements for the basic safety and essential performance of transcutaneous partial pressure monitoring equipment”
IEC 60601-2-23 Scope
Replacement:
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of TRANSCUTANEOUS PARTIAL PRESSURE MONITORING EQUIPMENT as defined in 201.3.63 and hereinafter referred to as ME EQUIPMENT, whether this ME EQUIPMENT is stand-alone or part of a system.
This standard applies to transcutaneous monitors used with adults, children, and neonates, and it includes the use of these devices in fetal monitoring during birth.
This standard does not apply to hemoglobin saturation oximeters or to devices applied to surfaces of the body other than the skin (for example conjunctiva, mucosa).
If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case, the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS, as relevant.
HAZARDS inherent in the intended physiological function of ME EQUIPMENT or ME SYSTEMS within the scope of this standard are not covered by specific requirements in this standard except in 7.2.13 and 8.4.1 of the general standard.
NOTE See also 4.2 of General Standard.
201.4.101 Additional ESSENTIAL PERFORMANCE requirements
Additional ESSENTIAL PERFORMANCE requirements are found in the subclauses listed in Table 201.101.
Table 201.101 – Distributed ESSENTIAL PERFORMANCE requirements
Requirement Subclause
Non-linearity and hysteresis 201.12.1.101.1
Time to alarm for pO2 and pCO2 ALARM CONDITIONS 208.6.6.1.103
201.17 ELECTROMAGNETIC COMPATIBILITY OF ME EQUIPMENT and ME SYSTEMS
Clause 17 of the general standard applies, except as follows:
Addition:
Clause 202 of this particular standard applies.
202 Electromagnetic compatibility – Requirements and tests
IEC 60601-1-2:2007 applies, except as follows:
202.5.2.2.2 Requirements applicable to ME EQUIPMENT and ME SYSTEMS other than those specified for use only in a shielded location
Addition:
ME EQUIPMENT and its ACCESSORIES shall not be considered LIFE-SUPPORTING ME EQUIPMENT.
202.6 ELECTROMAGNETIC COMPATIBILITY
202.6.1 EMISSIONS
202.6.1.1.2 Tests
a) *PATIENT cables
Replacement:
ME EQUIPMENT shall be tested with the TRANSDUCER with cable as specified by the MANUFACTURER with all SIP/SOP cables connected to ME EQUIPMENT (see Figure 202.101). The distances of SIP/SOP cables between the open end and floor (ground plane) shall be ≥ 40 cm.
Figure 202.101 – Set-up for radiated and conducted EMISSIONS testing according to 202.6.1.1.2 a)
202.6.2 * IMMUNITY
202.6.2.1.10 Compliance criteria
Addition:
The ME EQUIPMENT shall not change its operating state, shall not lose or change any stored data and, for pO2 in the range 40 mmHg (5,3 kPa) to 100 mmHg (13,3 kPa) and for pCO2 in the range 30 mmHg (4,0 kPa) to 60 mmHg (8,0 kPa), the change in the accuracy (ESSENTIAL PERFORMANCE) of the partial pressure reading shall not exceed ± 6 mmHg
(0,8 kPa).
Compliance is checked by the following test.
– Set up the ME EQUIPMENT and TRANSDUCER as outlined in Figure 202.101.
- Calibrate the TRANSDUCER according to the ACCOMPANYING DOCUMENTS.
- Expose the ME EQUIPMENT to the specified disturbances (radiofrequency, transients, magnetic) at any NOMINAL sensitivity.
202.6.2.2 ELECTROSTATIC DISCHARGE (ESD)
202.6.2.2.1 Requirements
Addition:
ME EQUIPMENT may show temporary DEGRADATION during discharges. Within 60 s the ME EQUIPMENT shall resume normal operation in the previous operating mode, without loss of any OPERATOR settings or stored data, and shall continue to perform its intended function and maintain ESSENTIAL PERFORMANCE (see 202.6.2.1.10).
202.6.2.3 Radiated RF electromagnetic fields
202.6.2.3.1 Requirements
Addition to item a):
IMMUNITY TEST LEVEL of 3 V/m applies.
202.6.2.3.2 Tests
Addition:
Test setup for radiated RF electromagnetic fields:
aa) Any SIGNAL INPUT PART/SIGNAL OUTPUT PART cable and POWER SUPPLY CORD arranged generally as in Figure 202.102. Maintain distances of ≥ 40 cm between SIP/SOP and the floor (ground plane).
Figure 202.102 – Set-up for radiated immunity test according to 202.6.2.3.2
202.6.2.4 Electrical fast transients and bursts
202.6.2.4.1 Requirements
Addition:
When exposed to electrical fast transients and bursts, via the POWER SUPPLY CORD, the ME EQUIPMENT shall continue to perform its intended function and maintain ESSENTIAL PERFORMANCE (see 202.6.2.1.10).
Testing of TRANSDUCER cables and interconnecting cables specified to be more than 3 m in length may show temporary DEGRADATION during exposure of fast transients and bursts. Within 60 s the ME EQUIPMENT shall resume normal operation in the previous operating mode, without loss of any OPERATOR settings or stored data, and shall continue to perform its intended function as described in the ACCOMPANYING DOCUMENTS.
202.6.2.4.2 Tests
Addition:
aa) ME EQUIPMENT shall be located 0,8 m ± 0,08 m above the reference ground plane.
bb) The power cord provided with the ME EQUIPMENT shall be used to connect ME EQUIPMENT to the output of the EFT/B generator.
202.6.2.6 Conducted disturbances, induced by RF fields
202.6.2.6.1 Requirements
Addition:
aa) When exposed to a conducted radio frequency voltage, via the POWER SUPPLY CORD, the ME EQUIPMENT shall continue to perform its intended function and maintain ESSENTIAL PERFORMANCE (see 202.6.2.1.10).
bb) * TRANSDUCER cables are exempt from this requirement.
202.6.2.6.2 Tests
Addition:
aa) Subclause 6.2.6.2, items c) and e) of IEC 60601-1-2:2007 do not apply.
F2 Labs can perform evaluations to IEC 60601-2-24 Edition 2.0 titled “Medical electrical equipment – Part 2-24: Particular requirements for the basic safety and essential performance of infusion pumps and controllers”
IEC 60601-2-24 Scope
Replacement:
This Particular Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of INFUSION PUMPS and VOLUMETRIC INFUSION CONTROLLERS hereafter referred to as ME EQUIPMENT.
This standard applies to ADMINISTRATION SETS insofar as their characteristics influence the BASIC SAFETY or ESSENTIAL PERFORMANCE of INFUSION PUMPS and VOLUMETRIC INFUSION CONTROLLERS. However, this standard does not specify requirements or tests for other aspects of ADMINISTRATION SETS
If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case, the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS, as relevant.
HAZARDS inherent in the intended physiological function of ME EQUIPMENT or ME SYSTEMS within the scope of this standard are not covered by specific requirements in this standard except in 7.2.13 and 8.4.1 of the general standard.
NOTE See also 4.2 of the general standard.
This particular standard specifies the requirements for ENTERAL NUTRITION PUMPS, INFUSION PUMPS, INFUSION PUMPS FOR AMBULATORY USE, SYRINGE, OR CONTAINER PUMPS, VOLUMETRIC INFUSION CONTROLLERS, and VOLUMETRIC INFUSION PUMPS, as defined in 201.3.204, 201.3.206, 201.3.207, 201.3.220, 201.3.222 and 201.3.223.
These particular standard does not apply to the following:
- devices specifically intended for diagnostic or similar use (e.g. angiography or other pumps permanently controlled or supervised by the OPERATOR);
- devices for extracorporeal circulation of blood;
- implantable devices;
- ME EQUIPMENT specifically intended for diagnostic use within urodynamics (a measurement of pressure-volume relationship of the urinary bladder when filled through a catheter with water);
- ME EQUIPMENT specifically intended for diagnostic use within male impotence testing (a measurement of the amount of liquid infused, necessary to maintain a preset pressure level for maintaining a penile erection: cavernosometry, cavernosography);
- devices covered by ISO 28620.
201.4.3 ESSENTIAL PERFORMANCE
Additional subclause:
201.4.3.101 Additional ESSENTIAL PERFORMANCE requirements
Additional ESSENTIAL PERFORMANCE requirements are found in the subclauses listed in Table 201.101.
F2 Labs can perform evaluations to IEC 60601-2-36 First Edition titled “Medical electrical equipment – Part 2: Particular requirements for the safety of equipment for extracorporeally induced lithotripsy”
IEC 60601-2-36 Scope
Addition:
This Particular Standard applies to the safety of EQUIPMENT FOR EXTRACORPOREALLY INDUCED LITHOTRIPSY as defined in 2.1.101.
The applicability of this Particular Standard is limited to components directly involved in the LITHOTRIPSY treatment, such as, but not limited to, the generator of the PRESSURE PULSE, PATIENT support device, and their interactions with imaging and monitoring devices. Other devices, such as PATIENT treatment planning computers, X-ray and ultrasonic devices, are excluded from this Standard because they are treated in other applicable IEC standards.
While this Particular Standard has been developed for EQUIPMENT intended for use in LITHOTRIPSY, it has been developed such that, as long as no other specific standards are available to be used for other medical applications of therapeutic extracorporeal PRESSURE PULSE equipment, this Standard may be used as a guideline.
F2 Labs can perform evaluations to IEC 60601-2-37 Edition 2.0 titled “Medical electrical equipment – Part 2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment”
IEC 60601-2-37 Scope
Addition:
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of ULTRASONIC DIAGNOSTIC EQUIPMENT as defined in 201.3.217, hereinafter referred to as ME EQUIPMENT.
If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case, the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS, as relevant.
HAZARDS inherent in the intended physiological function of ME EQUIPMENT or ME SYSTEMS within the scope of this standard are not covered by specific requirements in this standard except in 7.2.13 and 8.4.1 of this standard.
NOTE See also subclause 4.2 of this standard.
This particular standard does not cover ultrasonic therapeutic equipment. Equipment used for the imaging or diagnosis of body structures by ultrasound in conjunction with other medical procedures is covered.
201.4.3 ESSENTIAL PERFORMANCE
Addition:
201.4.3.101 Additional ESSENTIAL PERFORMANCE requirements
Table 201.102 lists the potential sources of unacceptable risk identified to characterize the ESSENTIAL PERFORMANCE of ULTRASONIC DIAGNOSTIC EQUIPMENT and the subclauses in which the requirements are found.
Table 201.102 – Distributed essential performance requirements
NOTE In some circumstances the need for the repetition of an ultrasound examination should be evaluated as a
potential hazard, for example, intra-corporeal investigation and stress testing for cardiopathic PATIENTS.
F2 Labs can perform evaluations to IEC 60601-2-39 Edition 2.0 titled “Medical electrical equipment – Part 2-39: Particular requirements for basic safety and essential performance of peritoneal dialysis equipment”
IEC 60601-2-39 Scope
Replacement:
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of PERITONEAL DIALYSIS ME EQUIPMENT as defined in 201.3.208, hereafter referred to as PD EQUIPMENT. It applies to PD EQUIPMENT intended for use either by medical staff or under the supervision of medical experts, including PD EQUIPMENT operated by the PATIENT, regardless of whether the PD EQUIPMENT is used in a hospital or domestic environment.
If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case, the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS, as relevant.
HAZARDS inherent in the intended physiological function of ME EQUIPMENT or ME SYSTEMS within the scope of this standard are not covered by specific requirements in this standard except in 7.2.13 and 8.4.1 of the general standard.
NOTE See also 4.2 of the general standard.
This standard can also be applied to PD EQUIPMENT used for compensation or alleviation of disease, injury, or disability.
These particular requirements do not apply to the DIALYSING SOLUTION or the DIALYSING SOLUTION CIRCUIT.
201.4.3 ESSENTIAL PERFORMANCE
Additional subclause:
201.4.3.101 Additional ESSENTIAL PERFORMANCE requirements
Additional ESSENTIAL PERFORMANCE requirements:
- DIALYSING SOLUTION flow to the patient;
- DIALYSING SOLUTION flow from the patient;
- the temperature of dialysate;
- adherence to and accuracy of the volume balancing (inflow/outflow volume).
F2 Labs can perform evaluations to IEC 60601-2-40 Edition 2.0 titled “Medical electrical equipment – Part 2-40: Particular requirements for the basic safety and essential performance of electromyography and evoked response equipment”
IEC 60601-2-40 Scope
Replacement:
This particular standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of ELECTROMYOGRAPHS and EVOKED RESPONSE EQUIPMENT. hereafter referred to as ME EQUIPMENT.
NOTE Myofeedback equipment, where the capturing of muscle contraction is based on electromyography, is within the scope of This particular standard. If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case. the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS. as relevant.
The following ME EQUIPMENT is excluded:
ME EQUIPMENT is intended for transcutaneous electrical nerve stimulators and electrical muscle stimulators (ME EQUIPMENT covered by IEC 60601-2-10.).
201.4.3 ESSENTIAL PERFORMANCE
Addition:
NOTE Because of the variety of clinical applications for ELECTROMYOGRAPHS and EVOKED RESPONSE, no additional
ESSENTIAL PERFORMANCE is specified in this particular standard. However, ESSENTIAL PERFORMANCE shall be
determined by the manufacturer in accordance with the requirements of sub-clause 4.3 of the general standard.
202 * ELECTROMAGNETIC DISTURBANCES – Requirements and tests
IEC 60601-1-2:2014 applies except as follows:
202.8 Electromagnetic IMMUNITY requirements for ME EQUIPMENT and ME SYSTEMS
202.8.1 General
Addition:
202.8.1.101 * IMMUNITY pass/fail criteria
The IMMUNITY pass/fail criteria of the ME EQUIPMENT or ME SYSTEMS shall be one or more of the following as determined by the manufacturer.
- The ME EQUIPMENT or ME SYSTEMS shall maintain BASIC SAFETY and ESSENTIAL PERFORMANCE during and after the IMMUNITY test. No DEGRADATION of ESSENTIAL PERFORMANCE is allowed below a level specified by the manufacturer.
- The ME EQUIPMENT or ME SYSTEMS shall maintain BASIC SAFETY and ESSENTIAL PERFORMANCE during and after the IMMUNITY test. However, temporary loss of ESSENTIAL PERFORMANCE is allowed, provided it can be restored without manual intervention. No DEGRADATION of ESSENTIAL PERFORMANCE is allowed below a level specified by the manufacturer.
- The ME EQUIPMENT or ME SYSTEMS shall maintain BASIC SAFETY and ESSENTIAL PERFORMANCE during and after the IMMUNITY test. However, temporary loss of ESSENTIAL PERFORMANCE is allowed, provided it can be restored with manual intervention. No DEGRADATION of ESSENTIAL PERFORMANCE is allowed below a level specified by the manufacturer.
Compliance is determined as follows:
The manufacturer shall define a method for determining whether ESSENTIAL PERFORMANCE has been maintained during and/or after IMMUNITY tests as appropriate based on the manufacturer’s RISK ASSESSMENT. Where manual intervention is required to restore ESSENTIAL PERFORMANCE, the method shall be provided in the instructions for use.
Where automatic recovery or manual intervention is allowed (criteria B, C) to restore ESSENTIAL PERFORMANCE, the time necessary to achieve restoration shall be justified in the RISK MANAGEMENT FILE.
Maintenance of BASIC SAFETY shall be determined by inspection of the equipment after completion of the IMMUNITY tests and if necessary by performing the appropriate tests of the general standard.
An automatic re-start of electrical, visual, or auditory stimulation shall not occur. See also subclauses 201.11.8 and 201.16.8 of this document.
NOTE 1 Disturbance of the display during any test does not constitute a non-compliance with the requirements of
this document.
NOTE 2 Where the manufacturer has determined there is no ESSENTIAL PERFORMANCE, the ME EQUIPMENT or ME SYSTEMS can cease to function during and/or after the IMMUNITY test.
202.8.9 IMMUNITY TEST LEVELS
Addition:
The pass/fail criteria of 202.8.1.101 for tests specified in Table 4 of IEC 60601-1-2:2014 are as described in Table 202.101.
The pass/fail criteria of 202.8.1.101 for the tests specified in Table 5 of IEC 60601-1-2:2014 are as follows:
- Pass/fail criterion A or B or C apply to the professional health care environment;
- Pass/fail criterion A or B applies to the HOME HEALTHCARE ENVIRONMENT.
The pass/fail criteria of 202.8.1.101 for the tests specified in Table 6 of IEC 60601-1-2:2014 are as follows:
- Pass/fail criterion A or B or C apply to the professional health care environment;
- Pass/fail criterion A or B applies to the HOME HEALTHCARE ENVIRONMENT.
The pass/fail criteria of 202.8.1.101 for tests specified in Table 7 of IEC 60601-1-2:2014 are as described in Table 202.102.
The pass/fail criteria of 202.8.1.101 for tests specified in Table 8 of IEC 60601-1-2:2014- are as described in Table 202.103.
The pass/fail criteria of 202.8.1.101 for tests specified in Table 9 of IEC 60601-1-2:2014 are as follows:
- Pass/fail criterion A or B or C apply to the professional health care environment;
- Pass/fail criterion A or B applies to the HOME HEALTHCARE ENVIRONMENT.
F2 Labs can perform evaluations to IEC 60601-2-41 Edition 2.0 titled “Medical electrical equipment – Part 2-41: Particular requirements for the basic safety and essential performance of surgical luminaires and luminaires for diagnosis”
IEC 60601-2-41 Scope
Replacement:
This particular standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of SURGICAL LUMINAIRES AND LUMINAIRES FOR DIAGNOSIS hereafter referred to as ME EQUIPMENT.
This particular standard does not apply to
- headlights;
- endoscopes, laparoscopes, and their light sources, which are covered by IEC 60601-2-18;
- luminaires used in dentistry, which are covered by ISO 9680;
- luminaires for general purposes, which are covered by IEC 60598-2-1 and IEC 60598-2-4;
- luminaires dedicated to therapeutic purposes;
- special-purpose lights with different conditions of use such as UV lights for dermatological diagnosis, slit lamps for ophthalmology, lights for surgical microscopes, and lights for surgical navigation systems;
- lights connected to surgical instruments;
- luminaires of emergency lighting, which are covered by IEC 60598-2-22.
NOTE See also 4.2 of the general standard.
201.4.3 ESSENTIAL PERFORMANCE
Addition:
The ESSENTIAL PERFORMANCE is the delivery of illumination and the limitation of energy to the operating field.
Requirement Subclause
DELIVERY OF A MINIMUM AND ADEQUATE ILLUMINATION ON THE OPERATING FIELD 201.12.1.102.1.1 a)
and 201.12.1.102.4
LIMITATION OF ENERGY IN THE OPERATING FIELD 201.12.1.102.1.1 a) 201.10.7 and
201.12.1.102.3
201.17 Electromagnetic compatibility of ME EQUIPMENT and ME SYSTEMS
Clause 17 of the general standard applies.
F2 Labs can perform evaluations to IEC 60601-2-46 Edition 2.0 titled “Medical electrical equipment – Part 2-46: Particular requirements for the basic safety and essential performance of operating tables”
IEC 60601-2-46 Scope
Replacement:
This particular standard specifies safety requirements for OPERATING TABLES, whether or not having electrical parts, including TRANSPORTERS, used for the transportation of the tabletop to or from the base or pedestal of an OPERATING TABLE with the detachable tabletop.
NOTE See also 4.2 of General Standard.
This particular standard does not apply to:
- dental patient chairs;
- examination chairs and couches;
- patient-supporting systems of diagnostic and therapeutic devices;
- OPERATING TABLE heating blankets;
- patient transfer equipment;
- delivery tables and beds;
- medical beds;
- field tables.
NOTE If OPERATING TABLES will be used in combination with diagnostic and/or therapeutic devices the relevant requirements of each particular standard have to be considered.
201.4.3 Essential performance
Addition:
Besides the definition of the MANUFACTURER, the following ESSENTIAL PERFORMANCE is required from OPERATING TABLES:
– no unwanted movement in any SINGLE FAULT CONDITION and any combined fault conditions
as derived from RISK MANAGEMENT specified by the MANUFACTURER.
201.12 Accuracy of controls and instruments and protection against hazardous outputs
Clause 12 of the general standard applies.
201.17 Electromagnetic compatibility of ME EQUIPMENT and ME SYSTEMS
Clause 17 of the general standard applies.
202 Electromagnetic compatibility – Requirements and tests
IEC 60601-1-2:2007 applies, except as follows:
202.6.2.2.1 Requirements
Replacement:
ME EQUIPMENT shall comply with the requirements of 6.2.1.10 [of IEC 60601-1-2:2007] as modified below. For this requirement, the following conditions associated with BASIC SAFETY and ESSENTIAL PERFORMANCE shall apply:
No permanent DEGRADATION or loss of function or OPERATOR settings that are not recoverable shall be observed at any immunity test level.
No inappropriate movement shall occur at all immunity test levels.
At all immunity test levels, the ME EQUIPMENT shall maintain ESSENTIAL PERFORMANCE within the specification limits.
At all immunity test levels, the temporary DEGRADATION or loss of function or performance is acceptable.
Within 10 s or after OPERATOR intervention without requiring the use of a tool, the ME EQUIPMENT shall resume normal operation in the previous operating mode, without loss of any OPERATOR settings or stored data, and shall continue to perform its intended function as described in the ACCOMPANYING DOCUMENTS.
Check compliance by application of the tests in 6.2.2.2. Evaluate the response of the ME EQUIPMENT or ME SYSTEM during and after these tests in accordance with 6.2.1.10 [of IEC 60601-1-2:2007] as modified above, considering each discharge individually.
Additional subclause:
202.6.2.2.1.101 Interference with high-frequency surgical equipment
OPERATING TABLES and remote control devices for OPERATING TABLES shall not present a HAZARDOUS SITUATION when used together with high-frequency surgical equipment.
Compliance is checked by the following tests:
NOTE 1 To accommodate the huge variety of high-frequency surgical equipment, two different test scenarios have been created.
- The high-frequency surgical equipment which is used for this test shall comply with IEC 60601-2-2, shall have a rated output power of 300 W at least for an impedance between 200 Ohms and 500 Ohms, a quasi-square wave output frequency characteristic, and shall operate in the frequency range of 400 kHz to 1 MHz.
- The high-frequency surgical equipment which is used for this test shall comply with IEC 60601-2-2, shall have an argon plasma coagulation mode with a peak voltage of 4 000 Vp (open circuit voltage) and 120 W power capability
NOTE 2 For details, see Annex A.
In all cases shall leads of the active and neutral electrodes be draped along the side rails and/or the exposed metal parts of the OPERATING TABLE top. The high-frequency surgical equipment shall then be operated in a mode that generates
an output power of 300 W (“conventional”) or 4 000 Vp/120 W (argon plasma coagulation).
c) Compliance
1) Operating the high-frequency surgical equipment at an open circuit shall cause no movement of the OPERATING TABLE.
2) Operating the high-frequency surgical equipment while short-circuiting the active and neutral electrodes and sparking with the active electrodes at the side rails and/or the exposed metal parts of the OPERATING TABLE top, shall cause no movement of the OPERATING TABLE.
NOTE 3 If operating tables will be used in combination with diagnostic X-ray equipment, the relevant
requirements of the collateral standard have to be considered.
F2 Labs can perform evaluations to IEC 60601-2-52 Edition 1.0 titled “Medical electrical equipment – Part 2-52: Particular requirements for the basic safety and essential performance of medical beds”
IEC 60601-2-52 Scope
Replacement:
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of MEDICAL BEDS intended for adults, hereafter referred to as MEDICAL BED as defined in 201.3.212.
If a clause or subclause is specifically intended to be applicable to a MEDICAL BED only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case, the clause or subclause applies both to MEDICAL BED and to ME SYSTEMS, as relevant.
HAZARDS inherent in the intended physiological function of MEDICAL BED or ME SYSTEMS within the scope of this standard are not covered by specific requirements in this standard except in 7.2.13 and 8.4.1 of the general standard.
NOTE See also 4.2 of General Standard.
201.12 Accuracy of controls and instruments and protection against hazardous outputs
Clause 12 of the general standard applies, except as follows:
201.12.2 USABILITY
Addition:
USABILITY analysis shall take into account MEDICAL BED design aspects such as the MATTRESS SUPPORT PLATFORM height, with regard to both OPERATOR and PATIENT, as relevant to the APPLICATION ENVIRONMENT.
NOTE Access to PATIENT for the purposes of medical treatment should not be delayed significantly by the SIDE RAILS and their respective control systems.
201.17 Electromagnetic compatibility of ME EQUIPMENT and ME SYSTEMS
Clause 17 of the general standard applies.
F2 Labs can perform evaluations to IEC 60601-2-57 Edition 1.0 titled “Medical electrical equipment – Part 2-57: Particular requirements for the basic safety and essential performance of non-laser light source equipment intended for therapeutic, diagnostic, monitoring and cosmetic/aesthetic use”
IEC 60601-2-57 Scope
Replacement:
This International Standard applies to BASIC SAFETY and ESSENTIAL PERFORMANCE of equipment incorporating one or more sources of OPTICAL RADIATION in the wavelength range 200 nm to 3 000 nm, with the exception of laser radiation, and intended to create non-visual photobiological effects in humans or animals for therapeutic, diagnostic, monitoring,
cosmetic/aesthetic or veterinary applications; hereafter referred to as light source equipment (LS EQUIPMENT).
This particular standard does not apply to equipment for sun tanning, ophthalmic instruments, or infant phototherapy.
NOTE Safety requirements in this particular standard are intended to address only HAZARDS to the eye and skin; hazards to internal tissues are not included in its scope.
LS EQUIPMENT may consist of single or multiple sources of OPTICAL RADIATION, with or without power supply, or may be incorporated into a complex system that includes optical, electrical, or mechanical systems or sources of other radiation.
NOTE Annexes AA to EE have been included for purposes of general guidance and to illustrate many typical cases. However, the annexes should not be regarded as definitive or exhaustive.
201.4 General requirements
Clause 4 of the general standard applies.
F2 Labs can perform evaluations to IEC 62366 Edition 1.1 titled “Medical devices – Application of usability engineering to medical devices”
IEC 62366 Scope
This International Standard specifies a PROCESS for a MANUFACTURER to analyze, specify, design, VERIFY and VALIDATE USABILITY, as it relates to SAFETY of a MEDICAL DEVICE. This USABILITY ENGINEERING PROCESS assesses and mitigates RISKS caused by USABILITY problems associated with CORRECT USE and USE ERRORS, i.e. NORMAL USE. It can be used to identify but does not assess or mitigate RISKS associated with ABNORMAL USE.
NOTE For the purposes of this standard, USABILITY (see 3.17) is limited to characteristics of the USER INTERFACE.
If the USABILITY ENGINEERING PROCESS detailed in this International Standard has been complied with and the acceptance criteria documented in the USABILITY VALIDATION plan have been met (see 5.9), then the RESIDUAL RISKS, as defined in ISO 14971, associated with USABILITY of a MEDICAL DEVICE are presumed to be acceptable, unless there is OBJECTIVE EVIDENCE to the contrary (see 4.1.2).
This International Standard does not apply to clinical decision-making relating to the use of a MEDICAL DEVICE.
F2 Labs can perform evaluations to IEC 80601-2-35 Edition 2.0 titled “Medical electrical equipment – Part 2-35: Particular requirements for the basic safety and essential performance of heating devices using blankets, pads or mattresses and intended for heating in medical use”
IEC 80601-2-35 Scope
Replacement:
This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of HEATING DEVICES using BLANKETS, PADS, or MATTRESSES in medical use, also referred to as ME EQUIPMENT. HEATING DEVICES intended to prewarm a bed is included in the scope of this International Standard.
If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case, the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS, as relevant.
If a clause or subclause is specifically intended to apply to a specifically defined type of ME EQUIPMENT, as is the case with FORCED AIR DEVICES, then the clause or subclause is entitled as such. Clauses or subclauses that apply to all types of ME EQUIPMENT within the scope of this standard are not specifically entitled.
HAZARDS inherent in the intended physiological function of ME EQUIPMENT or ME SYSTEMS within the scope of this standard are not covered by specific requirements in this standard except in 7 .2.13 and 8.4.1 of the general standard.
NOTE See also 4.2 of the general standard.
This particular standard does not apply to:
- HEATING DEVICES intended for physiotherapy; radiant warmers; for information, see IEC 60601 -2-21 (12]2; incubators; for information, see I EC 60601-2-19 (1 OJ;
- transport incubators, for information, see I EC 60601-2-20 (11 ];
- cooling devices.
201.4.3 ESSENTIAL PERFORMANCE
Addition:
201.4.3.101 Additional requirements for ESSENTIAL PERFORMANCE
Additional ESSENTIAL PERFORMANCE requirements are found in the subclauses listed in Table 201.101.
Requirement Subclause
Essential Performance requirement 1 201 .12.4.104 or generation of a TECHNICAL
ALARM CONDITION in compliance with
201 .12.3.103
F2 Labs can perform evaluations to ISO 14708-1 titled “Implants for surgery – Active implantable medical devices – Part 1: General requirements for safety, marking and for information to be provided by the manufacturer”
ISO 14708-1 Scope
This part of ISO 14708 specifies requirements that are generally applicable to ACTIVE IMPLANTABLE MEDICAL DEVICES.
NOTE 1 For particular types of ACTIVE IMPLANTABLE MEDICAL DEVICES, these general requirements are supplemented or modified by the requirements of particular parts of ISO 14708.
The tests that are specified in ISO 14708 are type tests and are to be carried out on samples of an ACTIVE IMPLANTABLE MEDICAL DEVICE to show compliance.
This part of ISO 14708 is applicable not only to active implantable medical devices that are electrically powered but also to those powered by other energy sources (for example by gas pressure 01′ by springs).
This part of ISO 14708 is also applicable to some non-implantable parts and accessories of the ACTIVE IMPLANTABLE MEDICAL DEVICES.
NOTE 2 The device that is commonly referred to as an ACTIVE IMPLANTABLE MEDICAL DEVICE can be a single device, a combination of devices, 01′ a combination of a device or devices, and one or more accessories. Not all of these parts are required to be either partially or totally implantable, but there is a need to specify some requirements of non-implantable parts and accessories if they could affect the safety or performance of the implantable device.
NOTE 3 In this part of ISO 14708, terms printed in small capital letters are used as defined in Clause 3. Where a defined term is used as a qualifier in another term, it is not printed in small capital letters unless the concept thus qualified is also defined.
NOTE 4 The terminology used in this part of ISO 14708 is intended to be consistent with the terminology of ISO/TR 14283:2004.
F2 Labs can perform evaluations to EN 45502, titled “Implants for surgery – Active implantable medical devices – Part 1: General requirements for safety, marking and for information to be provided by the manufacturer” EN 45502 ScopeThis part of EN 45502 specifies requirements that are generally applicable to ACTIVE IMPLANTABLE MEDICAL DEVICES.
NOTE 1 For particular types of ACTIVE IMPLANTABLE MEDICAL DEVICES, these general requirements are supplemented or modified by the requirements of particular standards which form additional parts of this European Standard.
The tests that are specified in EN 45502 are type tests and are to be carried out on samples of an ACTIVE IMPLANTABLE MEDICAL DEVICE to show compliance.
This part of EN 45502 is applicable not only to ACTIVE IMPLANTABLE MEDICAL DEVICES that are electrically powered but also to those powered by other energy sources (for example by gas pressure or by springs).
This part of EN 45502 is also applicable to some non-implantable parts and accessories of the ACTIVE IMPLANTABLE MEDICAL DEVICES.
NOTE 2 The device that is commonly referred to as an ACTIVE IMPLANTABLE MEDICAL DEVICE can be a single device, a combination of devices, or a combination of a device or devices and one or more accessories. Not all of these parts are required to be either partially or totally implantable, but there is a need to specify some requirements of non-implantable parts and accessories if they could affect the safety or performance of the implantable device.
NOTE 3 In this part of EN 45502, terms printed in small capital letters are used as defined in Clause 3. Where a defined term is used as a qualifier in another term, it is not printed in small capital letters unless the concept thus qualified is also defined.
ISO 7176-21:2009 Wheelchairs – Part 21: Requirements and test methods for electromagnetic compatibility of electrically powered wheelchairs and scooters, and battery chargers
ANSI/RESNA WC-2 2009, Section 21: Requirements and test methods for electromagnetic compatibility of electrically powered wheelchairs and motorized scooters
“These standards apply to electrically powered wheelchairs and scooters with a maximum speed of not more than 15 km/h intended for indoor and/or outdoor use by people with disabilities. They are also applicable to manual wheelchairs with an add-on power kit.
“They are not applicable to vehicles designed to carry more than one person. These parts also specify requirements and test methods for the electromagnetic compatibility of battery chargers intended for use with electrically powered wheelchairs and scooters.”
* Please note: This is a partial list of particular and collateral standards.
Frequently Asked Questions for Medical Device Testing
What Services Does F2 Labs Offer for Medical Device Testing?
F2 Labs offers a full range of medical device EMC testing and certification services, including:
- EMC Testing
- Electrical Safety Testing
- Wireless Coexistence Testing
- AIM 7351731 Testing
- Immunity to Other Sources of RF Emitters Testing
What Types of Medical Devices Can F2 Labs Test?
F2 Labs is equipped to test an extensive variety of medical devices, ranging from:
- Advanced robotic surgical systems
- Over-the-counter devices
- Other innovative and essential medical technologies
How Can F2 Labs Assist with FDA Medical Device Approval?
F2 Labs is an FDA ASCA (Accredited Standards Conformity Assessment) accredited lab. Their services for FDA approval include:
Key Benefits:
- Summary Test Reports: Streamlined reports that meet FDA ASCA standards, reducing the need for full reports.
- Efficient Approval: Expedites the FDA approval process, saving you months of back-and-forth communication with FDA reviewers.
- Proven Acceptance: The FDA readily accepts these Summary Test Reports, minimizing requests for additional documentation.
What Standards Does F2 Labs Test Against for Medical Devices?
F2 Labs tests medical devices against a variety of international and industry-specific standards, including:
- IEC 60601 Series
- ISO 14708-1
- EN 45502
- Other particular and collateral standards as required
If your device needs testing against additional standards, F2 Labs can accommodate those requirements.
Can F2 Labs Assist with International Certification for Medical Devices?
Yes! F2 Labs supports international certification, including:
- CE Mark for Europe
- U.S. NRTL (UL) Certification
- Canadian SCC (CSA) Certification
- Submissions for Australia, Brazil, and other global markets
What is the Importance of EMC Testing for Medical Devices?
EMC testing is critical to ensure medical devices:
- Function correctly in environments with other electronic devices
- Do not cause or experience electromagnetic interference
- Provide reliable and safe performance essential for healthcare settings
How experienced is F2 Labs in Medical Device EMC Testing?
With over 30 years of experience, F2 Labs has:
- Deep expertise in navigating the complexities of medical device certification
- A proven track record of assisting clients in meeting regulatory requirements effectively
How Does F2 Labs Ensure Timely Certification for Medical Devices?
F2 Labs prioritizes efficiency by:
- Working closely with clients to streamline the certification process
- Offering a dedicated team to ensure testing and approval timelines are met
- Helping devices reach doctors and patients as quickly as possible
FDA & Medical Related Frequently Asked Questions
Does F2 Labs assist with MRI conditional assessments?
Why should I care about who is doing my testing?
It is the responsibility of manufacturers of devices (“device firms”) to qualify third parties that generate data and to ensure that all information submitted to the FDA is truthful and accurate. See also Fraudulent and Unreliable Laboratory Testing Data in Premarket Submissions: FDA Reminds Medical Device Manufacturers to Scrutinize Third-Party-Generated Data | FDA.
Why would I not want to go with the “least expensive” option for testing?
Choosing a test lab based on price alone can backfire and end up costing time and money if the testing is not done properly. Additionally, in recent years, the FDA has observed that an increasing number of entities that contract with device firms to conduct testing on medical devices (“third-party test labs”) are generating testing data that are fabricated, duplicated from other device submissions, or otherwise unreliable. The FDA has identified an increase in submissions containing unreliable data generated by third-party test labs from numerous such facilities based in China and India. See also Fraudulent and Unreliable Laboratory Testing Data in Premarket Submissions: FDA Reminds Medical Device Manufacturers to Scrutinize Third-Party-Generated Data | FDA
How can I tell if a 3rd party testing lab is trustworthy to use?
The FDA encourages device firms to partner with third-party labs that have been voluntarily accredited under the Accreditation Scheme for Conformity Assessment (ASCA) program, doing such does not substitute for conducting an independent assessment of all third-party data. Third Party labs that are part of the ASCA program, (such as F2 Labs), had to go through a thorough evaluation with the FDA to be approved. See also Fraudulent and Unreliable Laboratory Testing Data in Premarket Submissions: FDA Reminds Medical Device Manufacturers to Scrutinize Third-Party-Generated Data | FDA
What does it mean to be FDA exempt and why do I still need testing?
As medical devices develop safety history by being on the market, the FDA recognizes that some are just really safe. So, they made some medical devices exempt from 510k submissions. When a device is exempt, the FDA makes it very specific about what they can claim and what they cannot.
Being exempt from 510k doesn’t mean they are exempt from the FDA regulations though. For example, design control, quality management system, validation and verification are all still requirements.
In lieu of the 510k, they still must register their establishment as well as register and list their device.
It’s vague but CFR 21 subpart B (is the quality management system requirement, see below for link) – one thing that is required is verification and validation – so per FDA definition, verification is the safety and EMC testing that is required.
When they don’t do the testing even though they registered – you get what’s called a 483 that says these are the nonconformities – it’s a threat – FDA eventually can fine you if you don’t comply.
CFR – Code of Federal Regulations Title 21 (fda.gov) – Link to the FDA QMS regulations
What is Health Canada and how is it different than the FDA?
Canada’s “FDA” is Health Canada – very similar to the FDA in terms of structure and classification of devices.
IF A CLIENT HAS NOT COMPLETED THEIR INTERNAL BENCH/VERIFICATION TESTING (THIS MEANS THAT THEIR RISK FILE POINTS TO ACTIVITIES THAT ARE IN PROCESS/NOT YET COMPLETED), DOES THE 60601-1 EVAL NEED TO WAIT UNTIL THESE ARE COMPLETED?
No – the 60601-1 evaluation can proceed, regardless of where they are in their internal process. This would result in two possible scenarios:
- Most of the time the internal bench testing does not affect the labs safety results, so we would simply be ensuring that they have addressed it/plan to address it (their RMF might indicate “pending bench testing results”). In the end the responsibility is on the manufacturer (not the lab), to ensure this happens successfully.
- If their internal activities could affect how a particular requirement out of the standard is handled, then we would be able to proceed, but could not complete the report until we received the final results/updated RMF. An example of this: the device is intended to provide heat to a person, and the internal manufacturers testing being done is to justify being able to raise the limits beyond what the standard states, then obviously we would need to wait for that before we could call it good/complete our report.
I have a product that already has 510(k) approval. I am making a change to the device. How do I know when I need to resubmit my 510(k) or if I can just document the change?
There is an FDA guidance that provides criteria and decision flowcharts for companies to follow when they make changes to a 510K cleared device. FDA relies on the company to follow this guidance to make a decision on whether they submit a new 510K or document the change.
Documenting a change can mean 2 things:
- Use the change order process and just document the change there. An example of this would be the company has an end of life component that they have to replace but it does not effect the device or the intended use of the device, so the change order process is sufficient
- Write a letter to file. A letter to file is strictly internal and is never sent to the FDA. The FDA does review a letter to file during their routine facility inspections and it is possible that FDA will disagree with the decision and recommend a 510K. An example of a letter to file would be changing the needle diameter on an RF microneedling device to a smaller size, but not changing the overall output energy.
Regardless of how changes are documented, all the supporting tests must be complete and included in the change order or the letter to file, so even though an IEC test report is not submitted to FDA in a 510K, it is required for the letter to file.
ChatGPT said: What is the definition of a patient?
PATIENT is Living being (person or animal) undergoing a medical, surgical or dental PROCEDURE [IEC 60601-1:2005, definition 3.76] (note: This matches with the ANSI/AAMI standard for the USA, and the EN standard for Europe).
Do medical devices used on animals need to be considered for testing under IEC 60601-1?
If that device was used to clean an animal’s tooth in a vet’s office, would that be considered a medical device?
Yes, as that is a dental device being used on an Animal.
What are examples of the types of “animals” that would be considered “Patients”?
Any Mammals, Birds, Reptiles, Amphibians, Fish, etc.