The CE marking is placed on products after compliance with all applicable EU CE marking Directives. Some typical examples of CE marking Directives that F2 Labs provides technical support for are indicated below:
- Machinery Directive 2006/42/EC
- Low Voltage Directive 2014/35/EU
- Electromagnetic Compatibility Directive 2014/30/EU
- ATEX Directive 2014/34/EU
- Radio Equipment Directive 2014/30/EU
- Medical Devices Directive 93/42/EEC
The option for the appointing of an Authorized Representative is made available in all of the above indicated CE marking Directives, but it is not the same for each, and whether or not it is required can also be dependent upon the compliance path of the product. We will investigate and summarize these requirements for the above EU laws in the following article.
Machinery Directive 2006/42/EC
The Machinery Directive (MD) 2006/42/EC defines what an authorized representative is as it pertains to the Machinery Directive in Article 2 (j): ‘authorised representative’ means any natural or legal person established in the Community who has received a written mandate from the manufacturer to perform on his behalf all or part of the obligations and formalities connected with this Directive;
That is pretty broad. Essentially, it means that an Authorized Representative can be assigned all or some of the duties, as applicable. For the Machinery Directive, you could appoint someone to handle the whole compliance process, just a small part of it, or anything in between. An important note is that there must be a written mandate (legal agreement) between the parties.
Low Voltage Directive 2014/35/EU
The Low Voltage Directive (LVD) 2014/35/EU, Article 1 (4) indicates the definition:
(4) ‘authorised representative’ means any natural or legal person established within the Union who has received a written mandate from a manufacturer to act on his behalf in relation to specified tasks;
Note that the definition in the LVD is not as broad. The definition includes ‘specified tasks’ and not ‘all or part’ as in the MD.
Next we move to Article 7 of the LVD which addresses specifically the role of the Authorized Representative as it pertains to the Low Voltage Directive.

Immediately we see that Article 6 (1) is referenced. That is below:

It is worth noting that Article 3 and Annex I are the requirements for the manufacturer in the LVD to make the product safe for use, i.e., the testing and evaluation.
Additionally, the technical file is referenced in Article 7 and explicitly states that the Authorized Representative cannot be responsible for compiling that, but can be the holder of the Technical File. This layer of separation between the compliance responsibilities (that may be assigned) as an LVD Authorized Representative is in contrast to the myriad of duties assignable as an MD Authorized Representative. To highlight this, attention should now be drawn to the minimum duties for an LVD Authorized Representative. For that see (above) Article 7 (2.) – (a), (b), & (c).
Annex III of the LVD is the sole conformity route available to LVD products. Annex III (4) shows the requirement for a CE marking and the EU declaration of conformity:

You may add these responsibilities to the Authorized Representative mandate, if you choose, by way of Annex III (5):

Electromagnetic Compatibility Directive 2014/30/EU
The Electromagnetic Compatibility Directive (EMCD) 2014/30/EU also allows the use of an Authorized Representative and since the EMCD, like the LVD, is a New Legislative Framework Directive the definition and duties of an Authorized Representative are the same. The New Legislative Framework of 2008, or “NLF” as it is referred, is the collection of a number of Directives that were written in order to streamline the responsibilities that overlap among the various Directives it encompasses. The responsibilities of an Authorized Representative under the LVD and EMCD (and others) are an example of this. Article 7 in the LVD is repeated perfectly as Article 7 in the EMCD.
The EMCD, unlike the LVD, does have mechanisms for the involvement of a Notified Body in the additional conformity modules besides Module A, Internal Production Control. The other conformity modules are indicated in Annex III, Part A, Module B, EU-type examination and Module C: conformity to type based on internal production control. These two additional conformity modules, unavailable in the LVD, allow for the interface of the appointed Authorized Representative with a Notified Body who may be involved in the EMCD compliance process by lodging the application with the Notified Body on behalf of the manufacturer.
ATEX Directive 2014/34/EU
The Equipment and Protective Systems intended for use in Potentially Explosives Atmosphere Directive (ATEX) 2014/34/EU follows the same path as the EMCD previously referred to.
Regarding ATEX, the European commission has published a complimentary document, the purpose of which is to elaborate, in plain English, the intent of the requirements listed in the ATEX Directive 2014/34/EU. The link for this document is here (it is a direct download link) and it is a free download, like all of the European Commission documents pertaining to CE marking. The document is titled ATEX 2014/34 EU Guidelines and section 3.5, below, elaborates on the role of the Authorized Representative.

Note that there is another paragraph to section 3.5 which is not pasted above. That paragraph deals with the role of the Authorized Representative responsibilities for equipment already in the EU and having used the expired ATEX Directive 94/9/EC instead of 2014/34/EU. This will not be discussed in this article.
Radio Equipment Directive 2014/53/EU
The Radio Equipment Directive (RED) 2014/53/EU follows the same path as the EMCD ATEX Directive previously referred to.
Medical Device Directive 93/42/EEC
The Medical Devices Directive (MDD) 93/42/EEC has perhaps the most requirements and responsibilities as compared to the other Directives referenced in this article. A manufacturer of medical devices, with the intention of making their products available in the EU, should also read and understand the very comprehensive overhaul of the MDD with the amendment to the MDD in Directive 2007/47/EU. The MDD makes requirements for the involvement of an Authorized Representative and the amendment elaborates on these roles and adds more. See below for further explanation.
There are twenty mentions of the term “authorised representative” in Directive 2007/47/EC. This article will focus on the most important aspects of the amended requirements.
First, we note that the installation of a required Authorized Representative for manufacturers without a physical EU footprint is now codified in the MDD by the addition of recital (14), below.

The definition of Authorized Representative, as pertains to the MDD, is added to Article 1 (2.) (j).

Article 10 (a) (2.) mandates the requirement for an Authorized Representative, physically located in the EU when the manufacturer does not have an office or location in the EU.

The MDD 93/42/EC, Article 14 paragraph two, displayed below…
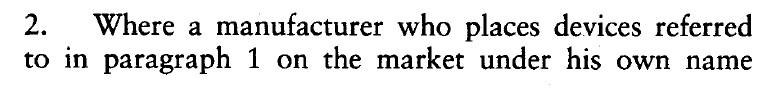

…is replaced by this section from the new Directive 2007/47/EC.

To sum this article up, please see the below bulleted points.
- The MD does not require an Authorized Representative but you may appoint one to handle some of the duties, some of which may be difficult to satisfy. For instance, the requirement for a European address on the declaration of conformity.
- The LVD, EMCD, ATEX, and RED do not require an Authorized Representative but if you assign one there is a minimum list of required duties that must be ascribed.
- The MDD requires an Authorized Representative if you are putting your products in the EU but do not have a physical location in the EU.
- All Authorized Representatives, for assistance in meeting compliance requirements for any of the aforementioned CE marking Directives, will require a written mandate or agreement in place that designates duties and responsibilities.
F2 Labs is here to help. Have a question or a comment? We can be contacted via this link. We can be reached by phone at 855-652-7281 and are here to help you.
