
It is safe to say that COVID-19 has affected everyone in the world. F2 labs immediately joined the fight and offered pro-bono services to our customers who are working to alleviate the suffering caused by this terrible illness.
“It was the right thing to do and, I am so proud of everyone on our team who worked to get these evaluations completed quickly and correctly,” is how Wendy Fuster, President of F2 Labs, characterized the effort.
One of our customers, Circadia Health, worked with F2 Labs in pursuit of FDA 510(k) clearance on a bedside device for monitoring the breathing of people stricken with the disease and to help with early detection.
Our engineers knew that this wasn’t just a project: it was a way for us to “get in the fight.”
This device is so significant that it was featured in today’s Wall Street Journal.
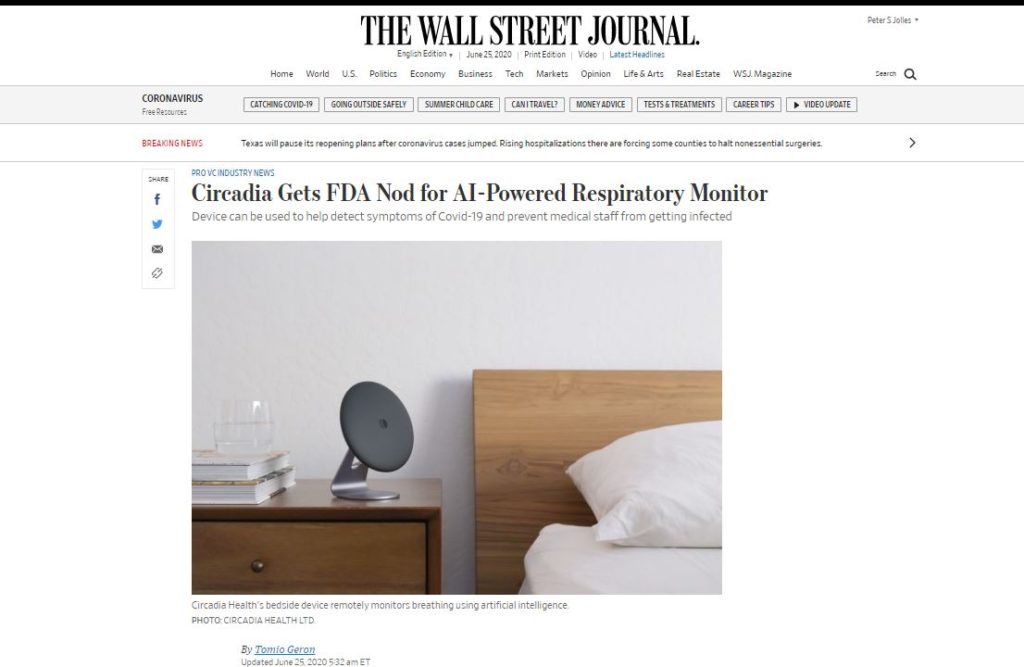
The Circadia C100 was evaluated at F2 Labs EMC and Safety testing laboratories.
We can be contacted via this link.
We can be reached by phone at 877-405-1580 and are here to help you.
